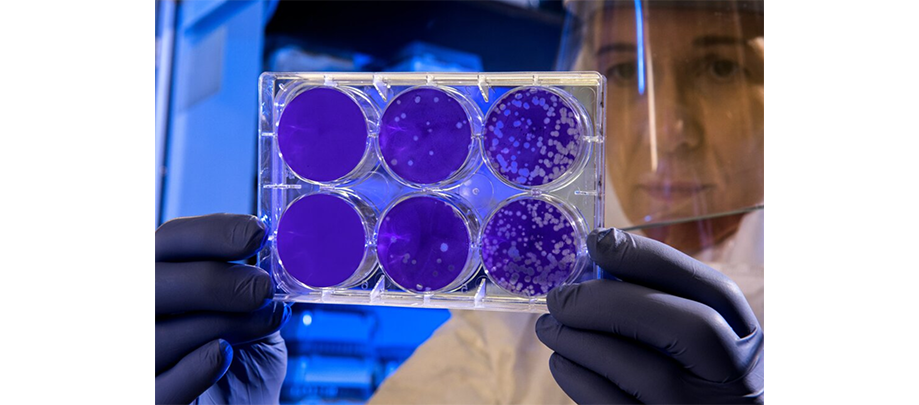
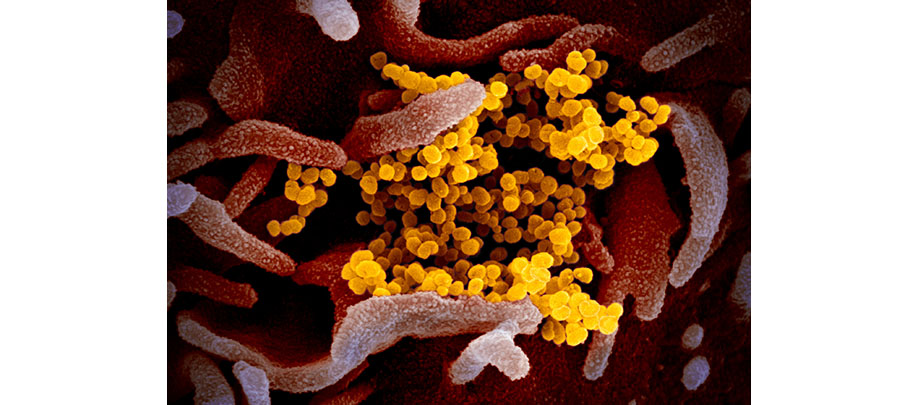
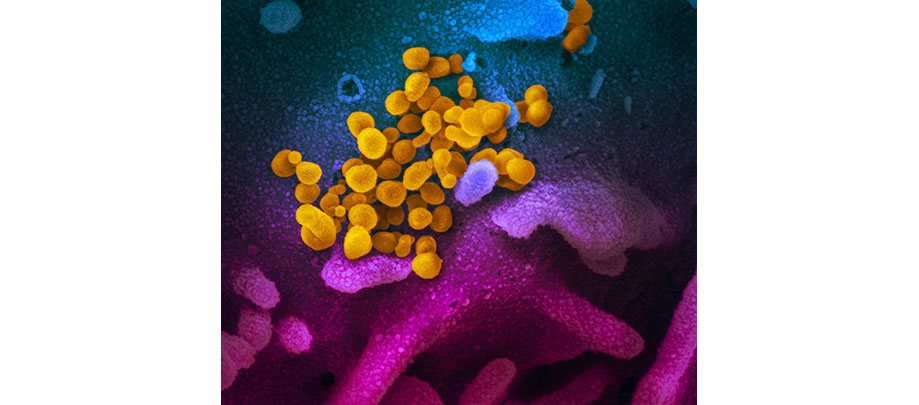
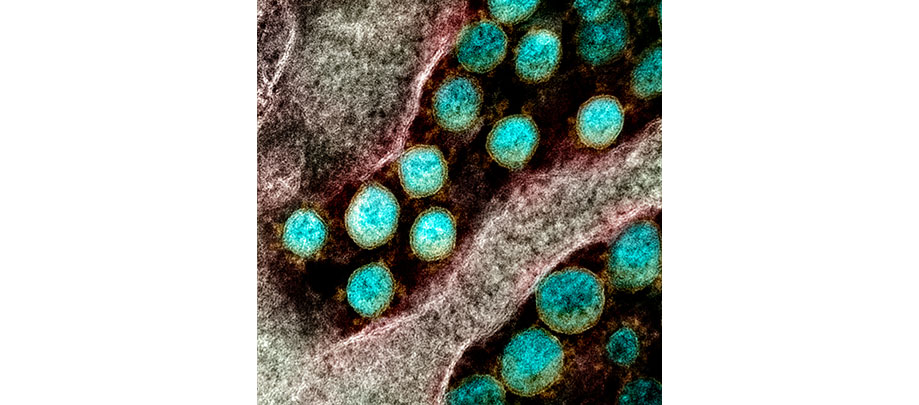
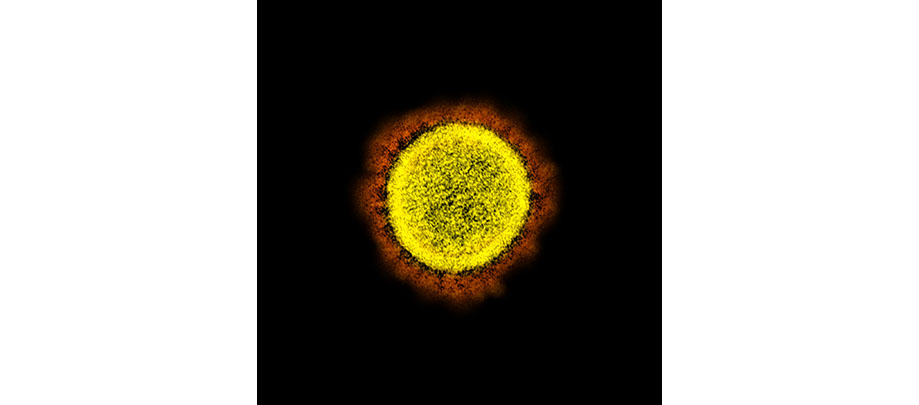
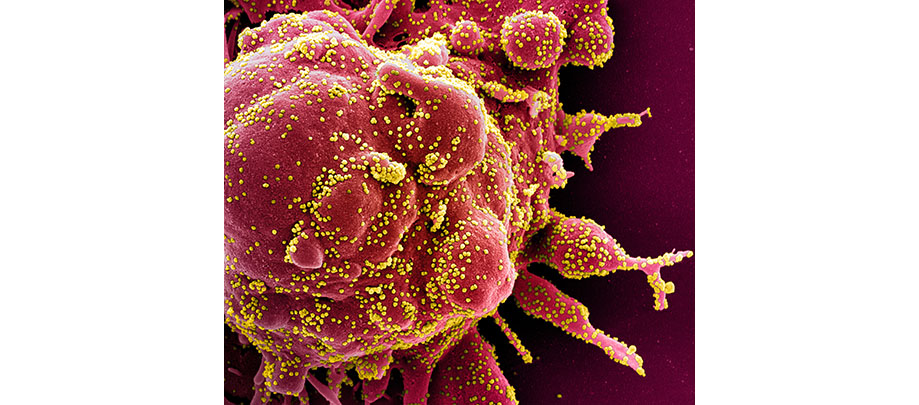
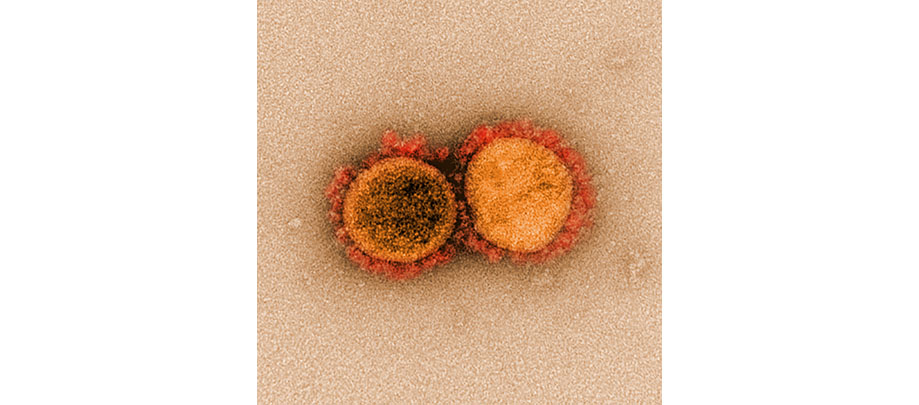
CLINICAL TRIALS
Coronavirus Research Institute is partnering with Biomed Industries, Inc. to conduct the following Phase 3 clinical trials: Phase 3 Clinical Trials of Biomedivir ™ for the prophylaxis and treatment of Covid-19CVIRI and Biomed are conducting two Phase 3 programs for Biomedivir ™: (1) The PREVENTION Program for first responders, ER doctors, nurses and other healthcare providers who are working closely with patients tested positive with Covid-19: Up to 300 healthcare providers/volunteers at 5 sites who will take Biomedivir over 4 weeks. (2) The TREATMENT Program for the patients with early onset with Covid-19 over 4 weeks. Up to 350 Patients at 5 sites will be selected within age group: 20-65 years of ages. 
Phase 3 Clinical Trials of Nanomedivir ™ for the prophylaxis and treatment of Covid-19CVIRI and Biomed are conducting two Phase 3 programs for Nanomedivir ™: (1) The PREVENTION Program for first responders, ER doctors, nurses and other healthcare providers who are working closely with patients tested positive with Covid-19: Up to 300 healthcare providers/volunteers will take Nanomedivir intranasal medication over 4 weeks. (2) The TREATMENT Program for the patients with early onset with Covid-19 over 4 weeks. Up to 350 Patients within age group: 20-65 years will take Nanomedivir intranasal medication over 4 weeks. These trials follow strict scientific standards and regulatory requirements to protect patients and help produce reliable results. Study physicians will review risks and potential benefits with patients and their caregivers before patients are enrolled in the clinical trials. The Phase 3 clinical trials have been designed to evaluate its safety and efficacy as required by the US Food and Drug Administration (FDA) and other regulators overseas before being approved for usage and marketing worldwide. Please contact us for study locations near you. Contact information is provided so you can reach out to them to find out more and schedule a visit. |